FDA partially halts leukemia studies of Gilead cancer drug
BioPharma Drive: Drug Pricing
AUGUST 21, 2023
The hold is the latest setback for a drug that was the center of Gilead’s $5 billion acquisition of biotech Forty Seven in 2020.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
AUGUST 21, 2023
The hold is the latest setback for a drug that was the center of Gilead’s $5 billion acquisition of biotech Forty Seven in 2020.
Drugs.com
MAY 22, 2024
WEDNESDAY, May 22, 2024 -- The number of American teens and young adults who've been prescribed one of the new GLP-1 weight-loss drugs soared nearly seven-fold between 2020 and 2023, a new report finds. That's compared to an overall decline of.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

BioPharma Drive: Drug Pricing
JULY 24, 2024
Known as SAGE-324, the drug was one of the key assets Biogen gained rights to through a billion-dollar research deal inked back in 2020.

Drug Patent Watch
DECEMBER 30, 2024
The regulatory environment in Japan for generic drug development is complex and has undergone significant changes in recent years. Regulatory Authority: Pharmaceuticals and Medical Devices Agency (PMDA) The PMDA is the primary regulatory authority responsible for overseeing the drug approval process in Japan.

Drug Channels
DECEMBER 14, 2021
This week, I’m rerunning some popular posts while I prepare for this Friday’s live video webinar: Drug Channels Outlook 2022. Since 2012, Drug Channels has examined commercial drug spending using the annual trend reports published by the largest PBMs. Specialty drug costs grew slowly—or even declined.

Common Sense for Drug Policy Blog
NOVEMBER 15, 2024
Misrepresentation of Drugs in the Unregulated Market "The misrepresentation of illicit drugs is a persistent problem in unregulated markets ( Barratt et al., When users consume illicit drugs of unknown content, quality, and dosage, their risk of overdose and other adverse health events increases significantly ( Singh et al.,
Eye on FDA
FEBRUARY 10, 2021
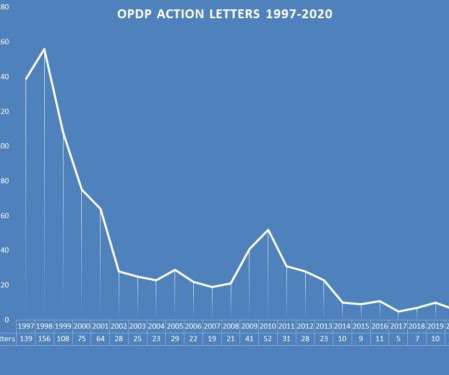
Looking now to a review of enforcement by the Office of Prescription Drug Promotion (OPDP) in 2020, we see a different picture. The collection of letters issued by OPDP during 2020 can be found here. The Violations – In recent years, there has been an uptick of activity aimed at the promotion of an unapproved drugs.

BioPharma Drive: Drug Pricing
SEPTEMBER 12, 2023
Johnson & Johnson and Bristol Myers Squibb have joined a group of new investors backing Rosana Kapeller’s startup, which launched in 2020 after incubating with GV.

Drug Patent Watch
JULY 28, 2023
A review article in the Journal of Pharmaceutical Sciences analyzes the trends in drug repurposing through the 505(b)(2) pathway, as approved by the USFDA from 2010 to 2020.
Drug Hunter
AUGUST 21, 2023
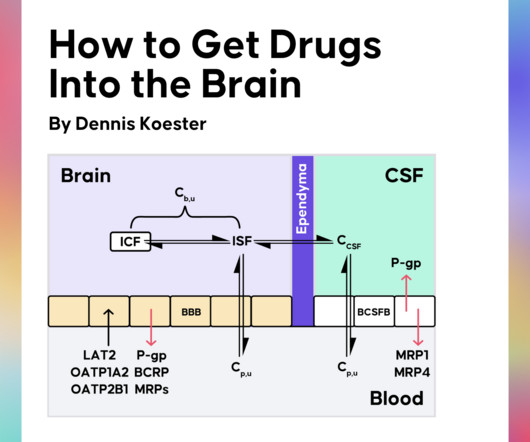
Obtaining adequate drug exposure in the brain is key to treating CNS diseases effectively. Recently, Dennis Koester gave us a crash course in CNS drug discovery in a Drug Hunter Flash Talk. Why Kp,uu is the Most Important Parameter in CNS Drug Discovery What Influences the Kp,uu of Drugs?

Drug Discovery Today
JUNE 10, 2020
For 2020 the BIO International Convention, usually one the world’s largest gatherings of the global biotech industry, is transitioning to a new, virtual event format. This is part of the main BIO programme which will be aired on Wednesday 10th June, with live Q&A.
addgene Blog
FEBRUARY 13, 2024
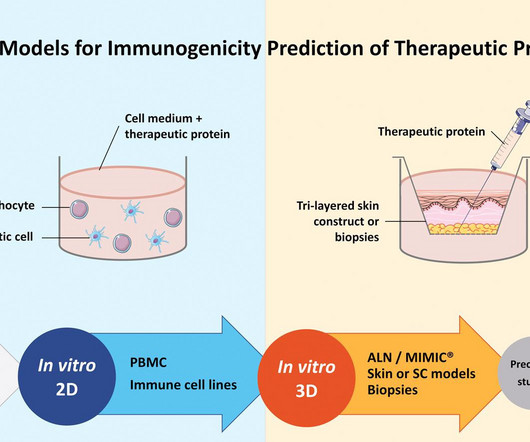
Monoclonal antibody drugs are popular therapeutics for a plethora of disease conditions, from cancer to autoimmune disorders. Antibodies administered as drugs are still immunogenic, meaning that they elicit an immune response from the body.
Drug Target Review
JUNE 9, 2025
A personal journey to Alltrna Michelle Werner’s career has spanned over 20 years in the pharmaceutical industry, where she developed her expertise in oncology drug development at leading companies such as Bristol Myers Squibb, AstraZeneca, and Novartis.
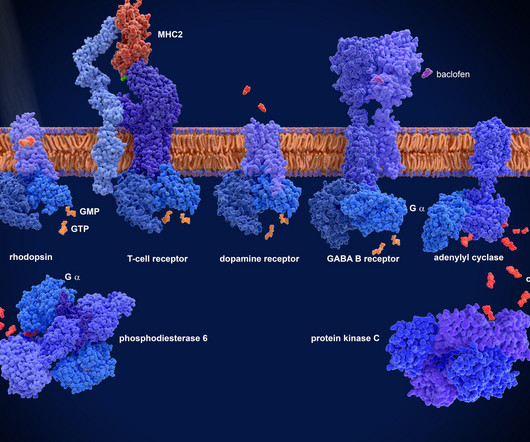
Drug Target Review
APRIL 7, 2025
The challenge of GPCR drug discovery G protein-coupled receptors (GPCRs) are one of the most desirable and challenging target classes in drug discovery, as their mutation can lead to a wide range of diseases such as cancer, cardiovascular disorders and neurological conditions.

Drug Channels
SEPTEMBER 24, 2021
This week, I’m rerunning some popular posts while I prepare for today's live video webinar: Drug Channels Update: Buy-and-Bill Market Trends. The Bureau of Labor Statistics (BLS) has recently released its employment and salary data for 2020. d/b/a Drug Channels Institute.
Drug Target Review
JULY 4, 2023
Over the last two decades, an increasing number of Antibody Drug Conjugate (ADC) therapeutics have been approved for oncology indications. These therapies have broadened treatment options for patients to expand beyond the more traditional small molecule drug alternatives. 3D rendering of Antibody Drug Conjugate Molecules.

The Pharma Data
NOVEMBER 1, 2020
Vancouver-based Algernon Pharmaceuticals plans to conduct a late-stage study to evaluate an orally delivered drug, NP-120 (ifenprodil), as a COVID-19 treatment. The drug was originally developed in the 1990s by Sanofi to treat peripheral circulatory disorders and it is still used in generic form in Japan. Source link.
Drug Target Review
SEPTEMBER 21, 2023
The findings supported the possibility put forward by Cotton et al (2016) concerning the chance of repurposing these drugs for adulticidal treatment. Need for adulticidal drugs More than 99 percent of countries worldwide has been affected by this disease. They provided a big stepping stone, needed for the development of drugs.

Drug Channels
SEPTEMBER 21, 2021
This week, I’m rerunning some popular posts while I prepare for this Friday’s live video webinar: Drug Channels Update: Buy-and-Bill Market Trends. In Drug Channels Institute's list of the top 15 pharmacies of 2020 , we show that many of the largest U.S. d/b/a Drug Channels Institute. Read on for all the details.
Drug Target Review
OCTOBER 30, 2024
Now take a step further: envision testing drugs in these organoids to identify the ones that can treat disease safely and effectively without needing to run expensive clinical trials first. 12 Testing drugs in vitro in organoid cultures A clear validation of the utility of organoids is in drug discovery and development.

Drug Patent Watch
JANUARY 7, 2025
Generic drugs play a crucial role in providing affordable medication options to patients. As healthcare professionals, it’s our responsibility to educate patients about generic drugs and empower them to make informed decisions about their treatment options. What Are Generic Drugs?
Broad Institute
JUNE 14, 2024
Some CRISPR screens may be missing cancer drug targets By Allessandra DiCorato June 14, 2024 Breadcrumb Home Some CRISPR screens may be missing cancer drug targets Current CRISPR guides don’t work equally well in cells from people of all ancestries, which could lead to false negative results.

Drug Target Review
MARCH 28, 2024
Between 2000 and 2020, approximately 30 percent of the newly introduced small molecule drugs were derived from natural products. In recent decades there has been a decline in interest in natural products for drug discovery, with the industry gravitating towards screening libraries of synthetic molecules with predefined chemistries.

Common Sense for Drug Policy Blog
JULY 16, 2023
US Cocaine Seizure Effectiveness Rate, 2016-2020 " Methodology and Limitations "Seizure data are obtained from OFO administrative records and is considered reliable. Drug Enforcement Agency’s National Drug Threat Assessment states that the Southwest Border remains the key entry point for the majority of the cocaine entering the United States.

PPD
DECEMBER 16, 2024
Our annual look at the state of the drug development industry highlights a dual set of challenges complicating progress. Rising costs have become a persistent challenge for drug developers, driven by a combination of internal and external pressures that have intensified in recent years.

Drug Patent Watch
DECEMBER 10, 2024
Generic drug development is a complex process that involves not only scientific and medical expertise but also adherence to strict legal and ethical standards. Scientific expertise plays a crucial role in ensuring that generic drugs are held to the same standards of quality, safety, and efficacy as their brand-name counterparts.

Drug Target Review
FEBRUARY 2, 2024
The standard animal model paradigm for drug discovery does not translate well to effects in patients for a number of reasons. In summary, all these factors can affect the response to drugs and drug target manipulation.

Drug Discovery Today
SEPTEMBER 23, 2020
Drug discovery expert leads not-for-profit organisation to drive further collaboration between academia and biopharma industry; Melanie to chair ELRIG Drug Discovery Digital 2020 from 6–16 October

Drug Discovery Today
JUNE 11, 2020
Webinar information:Join the experts on this free exclusive webinar on the 16th July 2020 at 2pm BST, hosted by West Pharmaceutical Services, Inc.

Drug Target Review
APRIL 7, 2025
In a recent survey conducted by ICON, Plc, biomarker selection was identified by 35 percent of respondents as a top challenge among drug developers for phase I trials, second only to navigating regulatory compliance (- 38 percent). To qualify as endpoints, biomarkers used in early phases must be relevant to later stages of drug development.

National Drug & Alcohol Research Centre Blog
DECEMBER 4, 2023
During the COVID-19 pandemic in 2020, long-acting injectable buprenorphine was recommended as a way for clients to continue on treatment whilst reducing social interactions. Our recent article, published in The International Journal of Drugs , presents the Australia-wide results. 2024) International Journal of Drug Policy.

FDA Law Blog: Biosimilars
OCTOBER 13, 2024
Designed to incentivize the development of drugs for pediatric rare diseases where such development may not otherwise have occurred, vouchers may be granted for drugs for serious or life-threatening rare diseases where the serious or life-threatening manifestations primarily affect individuals aged from birth to 18 years.

Drug Target Review
SEPTEMBER 26, 2023
These cells demonstrate considerable promise for uncovering drug-induced perturbations to neuronal function such as seizure, and their use extends further to sedation, anti-epileptic drug discovery and modelling of neurological diseases.

Drug Patent Watch
JANUARY 8, 2025
This comprehensive guide will walk you through the intricacies of optimizing your drug patent strategy, ensuring that your innovations are safeguarded and your market position is strengthened. Understanding the Basics of Drug Patents Before diving into the nuances of patent strategy, it’s crucial to grasp the fundamentals.

SCIENMAG: Medicine & Health
JULY 5, 2023
New drugs are often used not only for one disease (first approved indication) but also for other diseases (supplemental indications).
New Drug Approvals
JUNE 15, 2025
Retrieved 15 September 2024. ^ “Notice: Multiple additions to the Prescription Drug List (PDL) [2024-08-13]” Health Canada. Retrieved 15 August 2024. ^ “Regulatory Decision Summary for Zilbrysq” Drug and Health Products Portal. Food and Drug Administration (FDA). Food and Drug Administration (FDA).

Drug Channels
MARCH 8, 2022
Next week, Drug Channels Institute will release our 2022 Economic Report on U.S. It’s the 13th edition of our popular and comprehensive examination of the entire drug pricing, reimbursement, and dispensing system. d/b/a Drug Channels Institute. Drug Channels® is a registered trademark of Pembroke Consulting, Inc.
NIH Director's Blog: Drug Discovery
NOVEMBER 5, 2020
Imagine adding a different candidate drug to each well; then imagine adding the loaded quantum dots to each well and using machine vision to identify the wells where the dots could not enter the cell. 2020 Sep 22;14(9):12234-12247. Indeed, imagine thousands of tiny wells in which human cells are growing. That’s not science fiction.

The Premier Consulting Blog
APRIL 13, 2025
On April 10, 2025, the US FDA announced that it has a long-term plan to eliminate conventional animal testing in drug development, starting with monoclonal antibodies (mAbs).[ One great example is the FDAs 505(b)(2) New Drug Application (NDA) pathway. Legislation with delayed implementation In 2021, the FDA Modernization Act 2.0
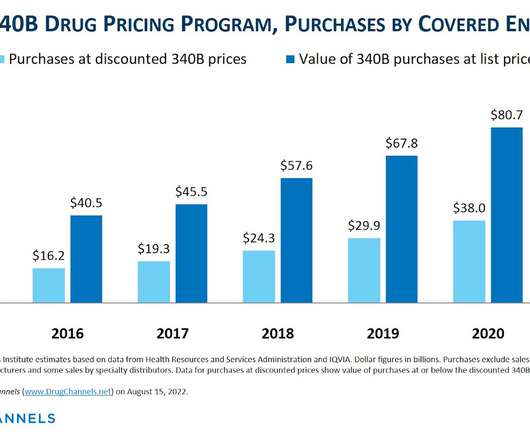
Drug Channels
AUGUST 15, 2022
Here’s a summer surprise for fans of the 340B Drug Pricing Program: Drug Channels has just obtained the 2021 figures from the Health Resources and Services Administration (HRSA)! billion (+15.6%) higher than its 2020 counterpart. billion (+15.6%) higher than its 2020 counterpart. d/b/a Drug Channels Institute.
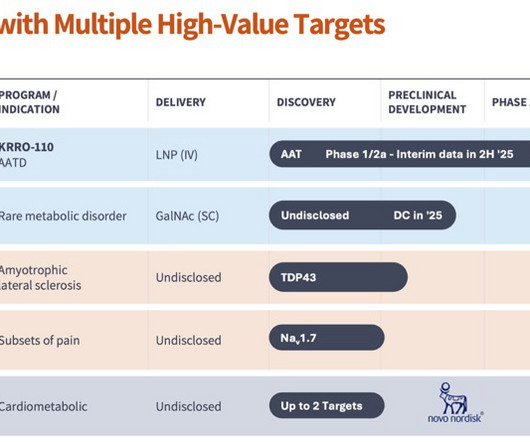
LifeSciVC
JUNE 12, 2025
Building medicines by activating biological pathways As I joined the Company in Q4 2020 as its CEO, I had a vision of creating protein variants to activate biological pathways. This meant focusing on clinical benefit for patients with AATD and build a drug candidate profile that was meaningful for patients.
Drug Target Review
SEPTEMBER 22, 2023
Organoid technologies are becoming an invaluable solution for preclinical research, with the ability to augment the development of personalised medicine, drug discovery and gene therapies. 5 Organoids are recognised as New Alternative Methods (NAMs) in drug development. billion by 2030.

FDA Law Blog: Biosimilars
JULY 11, 2023
Koblitz — One of the most important questions FDA has to answer is whether a given product is appropriately characterized as a drug, biologic, device, food, cosmetic, or something entirely different. the nanotube—is irrelevant, as it is the “active ingredient” that matters for purposes of classifying the product as a drug or biologic.
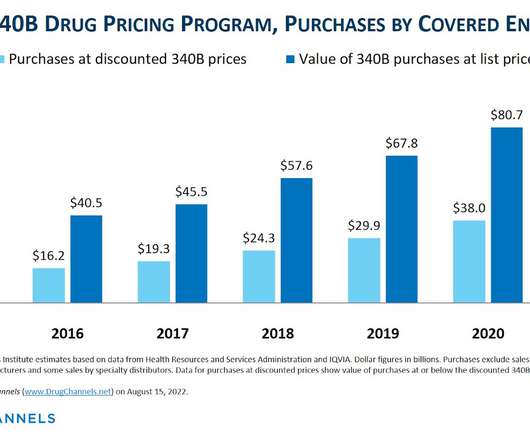
Drug Channels
DECEMBER 16, 2022
This week, I’m rerunning some popular posts while I prepare for today’s live video webinar: Drug Channels Outlook 2023. Here’s a summer surprise for fans of the 340B Drug Pricing Program: Drug Channels has just obtained the 2021 figures from the Health Resources and Services Administration (HRSA)! d/b/a Drug Channels Institute.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content